2014-06-04 Lachman CONSULTANTS
GDUFA实施的头两年中,仿制药办公室(OGD)DMF审评工作组一直在努力清理DMF审评积压,并试图解决2015年一旦进入GDUFA指标应如何有所准备。OGD不希望在DMF提交的基础上对GDUFA提交实施太严重的惩罚,因为GDFUA一生效就如此显著的影响和调整了DMF审评流程。因此,对于GDUFA的第1年和第2年,OGD在建立一个可行的系统上行使了一些监管的灵活性。现在OGD对ANDA申请人有一些建议,这些建议必须在申请提交之前纳入到您的研发和提交计划中。
最大的变化是,2015年财年(2014年10月1日)开始,在ANDA中引用到的任何II类DMF在ANDA提交时必须在“可供参考”清单中。ANDA立案审评开始时将检查这一清单。这与现行的等到立案审评结束时才确认DMF是否可供参考不同,现行的流程给了企业4个月的窗口期使得DMF通过完整性评估。
会上DMF审评工作组代表概括的其它要点如下:
- 书面形式提交的DMF可能花费企业额外7-10天的审评时间,因为DMF均是网站存储,以电子格式提交DMF更为重要。此外,以eCTD格式提交DMF可以提高效率并避免出错 — 提高首轮完整性评估的机会
- 在2007年之后收到完整科学审评的DMF大约需要20天的行政完整性评估。如果不确定您的旧DMF是否符合行政完整性评估请联系OGD – dmfogd@fda.hhs.gov
- 在ANDA提交6个月之前提交DMF并缴费,以便DMF有足够的时间通过完整性评估
- 现在OGD DMF工作组不完整行动的相应平均时间是45天 — OGD鼓励企业将相应时间减少到30天。
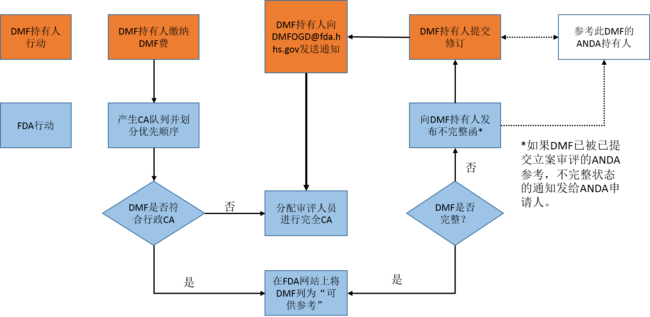
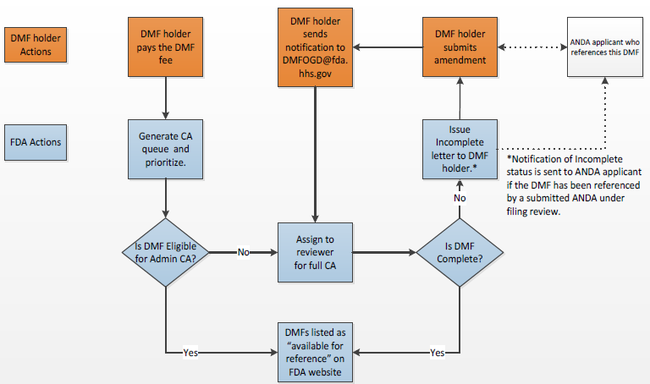
OGD概述的完整性评估流程如下图:
请注意,仅有18%的DMF在首轮审评周期收到完整性评估(CA)。约88%的DMF在第二轮审评周期收到CA。ECTD DMF在首轮收到CA的比率较高。 FDA列出了关于DMF的一些常见解释:
- API是一种物质,或者当该物质不稳定或无法依靠自身运输时可以是一种混合物。
- 混合物的DMF(例如,API加一种辅料)在GDUFA下可以作为API。我们要求当作此声明时,应提交相应理由到DMF。请注意,这些情况也有重要的设施费影响.
- CA要求DMF应为由单一生产工艺生产的单一API。
- 当DMF被提交时应开始稳定性研究并必须包含一个时间点通过的初始结果。
现在的DMF审评人员仅有不到39人,2013财年聘请了14人,2014财年聘请了25人。目标是到2014年末增长到61名DMF人员。
请注意OGD的建议并去参加一场会议以听取最近更新。不久,将收紧与参考清单可接受的DMF相关的立案审评。如果不这样做,可能导致拒绝接收,这一结果您应尽力避免,因为这将花去您25%的ANDA申请费并且可能给首次提交的机会带来风险。
Lachman CONSULTANTS - Terri Nataline 和Bob Pollock 2014-06-04
编译:识林-椒 2014-06-04
DMFs in the Spotlight at the GPhA/FDA CMC Workshop
Written by Terri Nataline and Bob Pollock • June 04, 2014
In the first two years of GDUFA, the Office of Generic Drugs (OGD) DMF review team has been hard at work clearing up the backlog of DMF reviews and trying to figure out how to stay ahead of the game once the GDUFA metrics kick in in 2015. OGD did not want to penalize GDUFA submissions based on DMF submissions too severely because the DMF review process was so dramatically impacted and adjusted once GDUFA went into effect. So for years 1 and 2 of GDUFA, OGD exercised some regulatory flexibility in establishing a workable system. Now OGD has some advice for ANDA applicants that must be incorporated into your development and submission plans before your application is submitted.
The big change is that, beginning in FY 2015 (October 1, 2014), any Type II DMF referenced in the ANDA must be on the "available for reference" list at the time of ANDA submission. The list will be checked at the beginning of the ANDA filing review. This differs from current practice of waiting until the end of filing review to confirm that the DMF is available for reference - which gave about a 4 month window for the DMF to undergo a completeness assessment.
Other nuggets outlined by a representative of the DMF Review Team during the presentation were:
- Submitting DMFs in paper format can cost the firm an extra 7-10 days of review time because the DMFs are stored off site-all the more important to submit the DMF in electronic format. Also, submitting the DMF in eCTD format improves efficiency and avoids errors - improving the chances of a first-cycle complete assessment
- DMFs that received a full scientific review after 2007 undergo an administrative complete assessment that takes approximately 20 days. If unsure whether your older DMF qualifies for an administrative complete assessment contact OGD - dmfogd@fda.hhs.gov
- Submit the DMF and pay the fee six months in advance of the ANDA submission to allow enough time for the DMF to undergo a complete assessment
- Average time to respond to an incomplete action by OGD’s DMF team is currently 45 days - OGD is encouraging industry to reduce response time to 30 days
The complete assessment process was outlined by OGD in the following diagram:
Note that only 18% of DMFs receive a complete assessment (CA) upon the first cycle of review. Approximately 88% of the DMFs receive a CA on the second cycle. ECTD DMFs have a higher rate of first-cycle CAs.
FDA outlined some common clarifications for DMFs:
- API is a substance or a mixture when the substance is unstable or cannot be transported on its own.
- DMF for a mixture (e.g. API plus an excipient) can qualify as the API under GDUFA. We ask that a justification be submitted to the DMF when this claim is made. Note that these situations also have important facility fee implications.
- The CA requires that a DMF be for a single API produced by a single manufacturing process.
- Stability studies must have started when the DMF is submitted and must contain one time point past initial results.
The current DMF review staff stands at just under 39, with 14 hired in FY 2013 and a 25 hired (so far) in FY 2014. The goal is to have a DMF staff of 61 by the end of 2014.
Pay attention to the advice of OGD and get out to a conference to hear the latest. The screws will be tightening on filing reviews relative to having DMFs in and on the acceptable for reference list shortly. Failure to do so may result in a refuse-to-receive, a result you should try hard to avoid as it will cost you 25% of the ANDA application fee and may put a first-to-file opportunity at risk. Please contact Terri Nataline at t.nataline@lachmanconsultants.com or one of our other regulatory experts at Lachman for any questions you may have regarding this process.
识林www.shilinx.com,版权所有,如需转载请注明出处