2014-06-08 Lachman CONSULTANTS
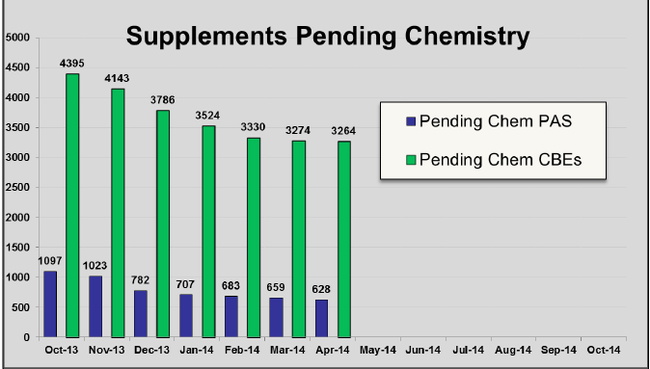
现在仿制药办公室(OGD)正在接受来自其他CDER部门的帮助努力减少补充申请积压。2013年5月,OGD报道仅有少于6000份补充申请积压。现在加上OGD朋友部门的帮助,总的补充申请积压已不到4000份。 OGD的Robert Lser给出的下图,表明了到2014年4月为止2014财年积压的下降。
我们所不知道的是,这些从OGD计时钟上移除的补充申请有多少是收到了完全回复函,又有多少得到批准。随着补充申请达到平均每月150份,OGD的统计报告不再提供补充申请完全回复函的数量或补充批准的数量。因此,不可能计算有多少补充申请会以新的面貌出现在未决队列中。
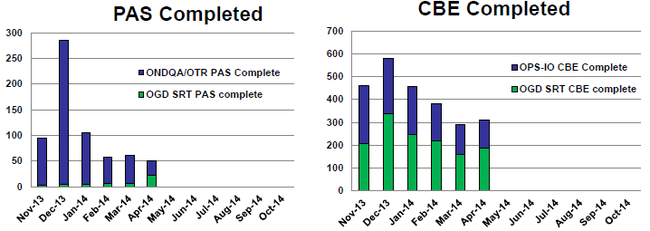
明显地,OGD已获得援助帮助自己度过补充申请积压的难关,为了从定量的角度让你更清楚,Robert Iser的另外一张图表讲述了一个有趣的故事。制药科学办公室成立的补充申请审评工作组专注于所有GDUFA之前的补充申请,而OGD的化学家们负责解决所有GUDFA之后补充申请。
祝OGD好运,继续成功地进行这方面的努力。我们希望在GPhA秋季技术研讨会上看到这一问题的进展,同时在研讨会上我们很可能看到本财年剩余的提交量和FDA在改变这些数字方面的行动。
Lachman CONSULTANTS - Bob Pollock先生 2014-06-05
编译:识林-椒 2014-06-08
Good News on Supplement Backlog Outlined at GPhA/FDA CMC Workshop
Written by Bob Pollock • June 05, 2014
The Office of Generic Drugs (OGD) is now receiving help from other CDER components in its battle to get the supplement backlog down. In May of 2013, OGD reported just fewer than 6000 supplements in its backlog. Today with a little help from their friends, the total supplemental backlog stands at less than 4000. The chart below prepared by OGD's Robert Iser illustrates the decline in the backlog in FY 2014 through April 2014.
What we don't know is how many of the actions that took the supplements off of the OGD clock were Complete Response letters versus approvals. With supplements arriving at an average of about 150 per month, the OGD-reported statistics do not provide the number of Complete Response letters to supplements or the number of supplemental approvals; thus, it is not possible to calculate how many of these supplements will make a new appearance in the pending column.
It is certainly clear that the assistance OGD has been getting is helping them turn the corner on the supplemental backlog and, to give you an idea of the help from a quantitative perspective, another one of Robert Iser’s charts tells an interesting story. Just know that the Office of Pharmaceutical Science-created Supplemental Review Team is concentrating on all pre-GDUFA supplements, while OGD chemists are tackling all post-GDUFA supplements.
Good luck, OGD, on continuing this effort successfully. We hope to see how this issue is progressing at the GPhA Fall Technical Workshop where we are likely to see how the rest of the fiscal year submissions and FDA actions change the numbers.
识林www.shilinx.com,版权所有,如需转载请注明出处